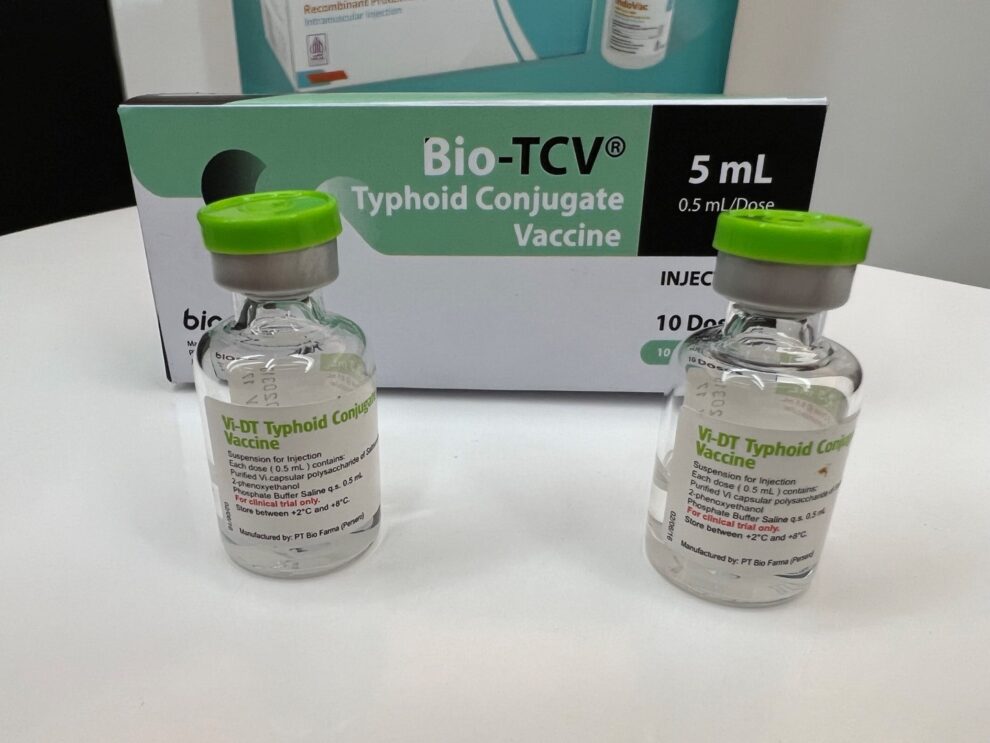
The International Vaccine INSTITUTE (IVI) announced that the typhoid conjugate vaccine (TCV), Bio-TCV, produced by PT Bio Farma, has received a distribution permit in Indonesia.
Bio Farma President Director Shadiq Akasya, in Bandung, Thursday (9/11) said that the licensing of the typhoid conjugate vaccine (Bio-TCV) from the Food and Drug Supervisory Agency (BPOM) was proof of Bio Farma’s commitment to global health in fighting infectious diseases. through the provision of safe vaccines.
“Bio TCV will be an important tool in preventing typhoid infection, providing protection against this disease from the age of nine months,” he said.
According to him, the successful development of Bio-TCV is proof of Bio Farma’s commitment to global health, fighting infectious diseases by providing safe and efficacious vaccines that meet international quality standards.
IVI and Bio Farma confirmed the safety and immunogenicity of a single dose of Vi-DT and that it was not inferior to the TCV control that had met WHO requirements in phase III clinical trials in three provincial capitals in Indonesia.
“With the results of this research, BPOM approved the vaccine for national use in individuals aged nine months to 45 years,” he said. Bio Farma, continued Shadiq, will submit documents for WHO PQ which, if achieved, will add TCV at affordable prices to the public market global.
The vaccine will be available to low-income countries through the Global Alliance Vaccine Initiative (GAVI).”Typhoid fever is a potentially life-threatening febrile illness caused by Salmonella typhi that primarily affects children and young adults.
“According to WHO, it is estimated that there are 11 to 20 million typhoid cases every year, most of which occur in low and middle income countries,” he explained.
Vaccination, he added, has been proven to be an effective prevention strategy in controlling typhoid fever, although there are only two vaccines that have been qualified by WHO at this time. IVI is working with vaccine manufacturers around the world to make more TCV available on the public market. (SG).
Source: Media Indonesia